The hydrocarbon methane is highly abundant on Earth, yet its release is now known to contribute to surges in temperature and climate change. In recent years, researchers have been trying to devise reliable methods to directly convert methane into other fuels and chemicals with valuable real-world applications.
These strategies include catalyst-based methods to prompt the oxidative coupling of methane to substances containing the green gas diatomic carbon (C2). This reaction, however, typically requires extreme and challenging environmental conditions, due to the unfavorable characteristics of the thermocatalysts introduced so far.
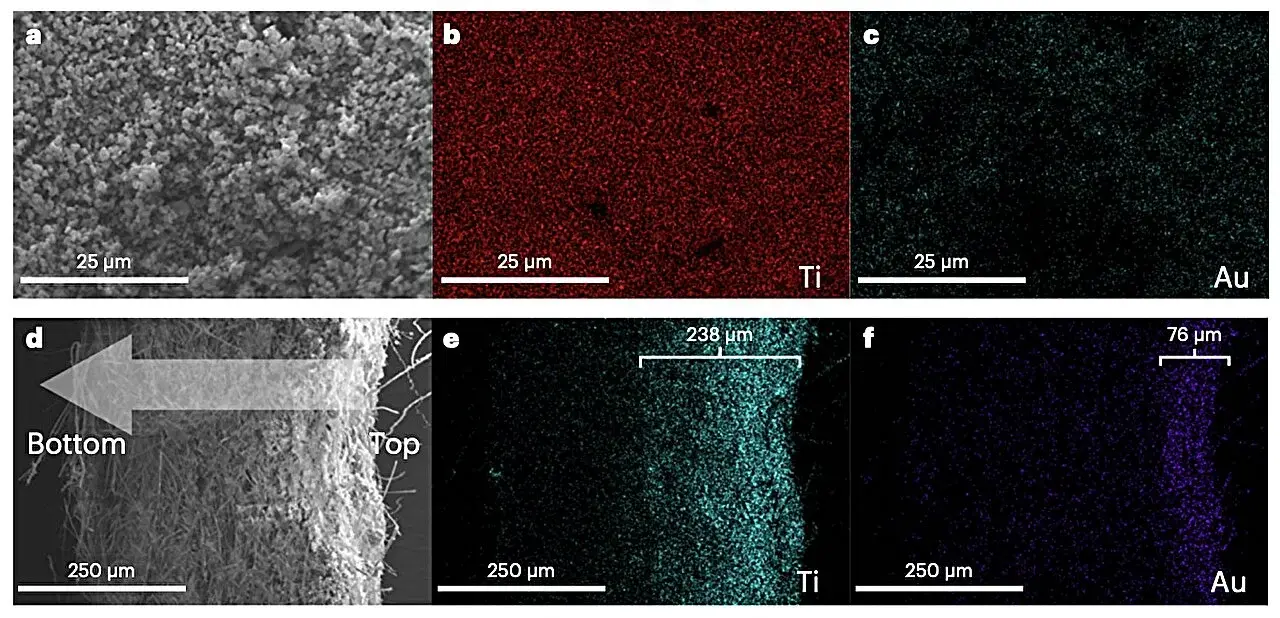
Researchers at University College London, University of Liverpool have recently developed a new photocatalyst that could advance the oxidative coupling of methane. This photocatalyst, introduced in Nature Energy, is based on titanium dioxide (TiO2 ) loaded with gold (Au) nanoclusters.
“Photocatalytic oxidative coupling of methane (OCM) produces C2 molecules that can be used as building blocks for synthesis of fuels and chemicals,” Xiyi Li, Chao Li and their colleagues wrote in the paper. “However, the yield rate and the selectivity of C2 products are still moderate due to the stable nature of methane molecules.”
Using a rapid sputtering method, the researchers were able to homogenously load Au nanoclusters onto TiO2, producing their promising new photocatalyst. In initial tests, an optimized sample of their photocatalyst appeared to perform remarkably well, converting methane into C2 at a high rate and without requiring particularly harsh reaction conditions.
“We develop an Au nanocluster-loaded TiO2 photocatalyst by a sputtering approach, achieving a high methane conversion rate of 1.1 mmol h−1, C2 selectivity of ~90% and apparent quantum efficiency of 10.3 ± 0.6%,” Xiyi Li, Chao Li and their colleagues explained in the study.
“The high C2/C2+ yield rate is on the same order of magnitude as the benchmark thermal catalysts in OCM processes operated at high temperature (>680 °C). Au nanoparticles are shown to prolong TiO2 photoelectron lifetimes by a factor of 66 for O2 reduction, together with Au acting as a hole acceptor and catalytic center to promote methane adsorption, C–H activation and C–C coupling,” they continued.
Overall, this study demonstrates the advantages of using catalysts based on various components with different functions and characteristics for enabling the oxidative coupling of methane. Their proposed photocatalyst, Au60s/TiO2, was found to outperform many previously reported catalysts that can trigger this reaction, in terms of stability, methane conversion rate and yield of C2.
Notably, the team’s photocatalyst is also easy to fabricate, which could facilitate its large-scale production and deployment. More studies could soon help to validate the performance of the new Au60s/TiO2 photocatalyst and assess its applicability in real-world settings.
In the future, this study could also pave the way for the fabrication of other promising multi-material photocatalysts for the reliable and direct conversion of methane. Collectively, these efforts could aid the valuable utilization of the abundant reserves of methane on Earth.